Mercury allergy
There are different forms of mercury, MELISA testing can differentiate between allergy to each form. Each type has its different properties and is therefore utilized in different ways in dentistry, medicine and industry or is present in the environment. Allergy to mercury is specific, so an individual can be allergic to inorganic mercury and not to phenylmercury. This is because the cells involved in Type IV allergic reaction – memory T cells – will recognise a specific form of mercury. Naturally, it is possible that an individual can be allergic to all four types of mercury. In many cases it is important to locate the source of exposure to mercury that causes inflammation in the body, so that exposure can be stopped.

Inorganic mercury, or ‘metallic mercury’, is a frequent source of metal allergy. It constitutes 50% of dental amalgam fillings. Dental authorities accept that mercury vapour constantly evaporates from the fillings, but argue this is below a safe limit. However, for hypersensitive patients, there is no safe limit.
In 2020 The FDA issued the following statement: “The FDA has found that certain groups may be at greater risk for potential harmful health effects of mercury vapor released from the device. As a result, the agency is recommending certain high-risk groups avoid getting dental amalgam whenever possible and appropriate.” For more information, please check HERE.
One of the groups at greater risk for potential harmful health effects is “People with known heightened sensitivity (allergy) to mercury or other components of dental amalgam.”
Replacing amalgam fillings with non-metallic alternatives has delivered radical health improvements in patients who tested MELISA-positive for mercury. 71% of patients showed health improvements in this study.

Methylmercury is the most toxic form of mercury. It affects the immune system, alters genetic and enzyme systems, and damages the nervous system, including coordination and the senses of touch, taste, and sight. Methylmercury is particularly damaging to developing embryos, which are five to ten times more sensitive than adults. Methylmercury becomes concentrated as it move up the food chain, so the large predator fish such as shark and swordfish have the highest concentrations. Bacteria in the body can transform inorganic mercury into methylmercury.

Phenylmercury is the organic mercury most commonly found in dental root fillings. While it has been phased out in many countries, it is also used as a preservative in eye drops and nose drops. Phenylmercury is used to control the growth of fungus in some interior latex paints manufactured before 1991, some exterior and oil base paints, some caulks, eye-area cosmetics, toiletries and other products.

Ethylmercury is a form of organic mercury. It forms a part of thimerosal, which is used as a preservative in some vaccines, eye drops and nasal sprays.

Thimerosal is one of the most controversial substances is modern medicine. Its main component is ethyl mercury (49.6% by weight), yet it is still used as a preservative in some childhood vaccines, most flu vaccines and some eye drops and contact lens solution. It is being withdrawn from vaccines in many countries.

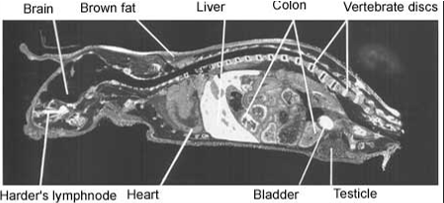
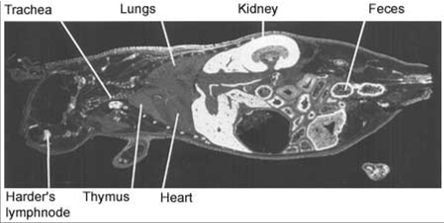
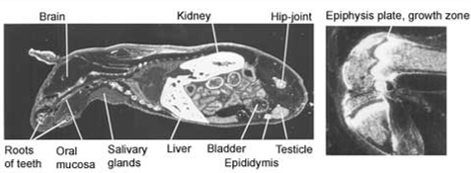
Full body autoradiograph
A special form of mercury can be used to demonstrate that mercury binds to the body proteins. The distribution of mercury in various organs of the body can be traced by sensitive photographic emulsion. Below are four pictures of mice that were injected with mercury labelled with a radioactive isotope. Then, using autoradiography, a special picture was produced. The areas where the mercury is deposited are shown in white.
The pictures demonstrate widespread distribution of mercury in the body of the mice. Organs rich in fat – such as brain and collagen – are very prone to mercury binding. One of the reasons for this is that mercury is particularly keen to bind to two amino acids; methionine and cysteine. Both amino acids contain sulphur hydrogen (SH)-groups. This is a particularly attractive target for mercury. Fat tissues and collagen tissues are rich in SH-groups.
|
Figure 1. Distribution of radioactivity in male mouse 6 hours after intravenous injection of 203HgCl2. Magnification 2x. |
|
|